
In the last two decades, computational neuroscience has experienced enormous development. This can be seen in the number of scientific publications that are produced in this discipline year after year. In the graph you can see how around 2007 the discipline began a constant rise that has not stopped to this day. Although today the number of publications per year has not reached as high number as other disciplines, its rise is abundantly increasing (Figure 1). This increase reflects the impact that computational neuroscience is having on different branches of knowledge in neuroscience, from the understanding of neuroimaging data, through intervention techniques such as neuromodulation, to influencing electronic systems and robotics.

In recent years, many books have been published in this discipline, and there are also specific scientific journals in this field (for example, PLoS Computational Biology, Frontiers in Computational Neuroscience, etc.).
The early scientific roots of the field can be traced to the work of Louis Lapicque (1907). He developed the Integrate and Fire neuron model, which is still popular today for key neural network studies due to its simplicity of understanding and the possibility of mass implementation. With a diametrically opposite approach, scientists Alan Lloyd Hodgkin and Andrew Huxley proposed in 1952 a very detailed model, composed of a set of differential equations that approximates the electrical characteristics of excitable cells such as neurons (thanks to which, they received the Nobel Prize of Physiology or Medicine in 1963).
One of the simulation scenarios most addressed in work in recent years consists of modeling the condition at rest, due to the ease of carrying out this type of study, and the possibility of comparison with similar studies that this simplified protocol entails (Figure 3).
Among the different simulation techniques, spiking neural networks (SNNs) allow detailed simulation of neuronal activity from the level of individual neurons to interconnected neuronal populations. Taking a different approach, neural mass models (NMMs) are suitable for simulating at the population level and not at the individual neuron level, but allow simulations of larger brain networks (up to the scale of the entire brain). with limited computing resources.
The two simulation techniques SNNs and NMMs are used in a complementary way, to understand and reproduce the mechanisms underlying diseases of the central nervous system, through the study and reproduction of typical patterns of functional connectivity (FC), and their disruption, obtained from real brain data (neurophysiological and structural).
The aim is to develop a Computational Neuroscience Institute with an international perspective to improve the understanding of brain mechanisms governing normal and pathological activity. Our main topics of study are threefold: Focus on neurodevelopmental pathologies, research into the keys to human intelligence and neuromodulation interventions. These three topics have not yet been the subject of major proposals and therefore this Institute could be a pioneer and generate leadership in these topics. Furthermore, the aim is to generate individualized models that can therefore be used in the clinical setting. Inspiration from neuro-robotics models will also be on the horizon of this Institute, although due to its complexity it will require collaboration with other researchers dedicated to mechanics and robotics.
The following lines of research are proposed:
Development of spiking network and neural masses models to understand the mechanisms of brain network alterations in children diagnosed with ADHD.
Development of spiking network and neural masses models to understand the mechanisms of brain network alterations in children diagnosed with autism.
Development of spiking network and neural masses models to understand the brain mechanisms that underlie human intelligence.
The Institute is equipped with advanced data computing technology (CPUs and GPUs) and access to a processing cluster. This facilitates data analysis processes and the execution of objectives. Likewise, brain activity recording (EEG, MEG; fMRI) and neuromodulation technologies are available, both to be able to evaluate the hypotheses generated in the computational models, and to generate, from the analysis of real data, hypotheses that will be subjected to validation through computational models.
The final objective of the development of these computational models is to find the optimal configuration and duration of the stimulation to be carried out in pathological subjects, to restore a functioning of the brain network that is as similar to that of healthy people. For this purpose, the in-silico designed interventions will be implemented in real subjects through non-invasive brain stimulation (NIBS). Recent advances in this field have provided accessible devices for a multitude of research purposes and have made neuromodulation techniques safer. In this sense, the scientific literature documents that transcranial electrical stimulation (tES) gives us the possibility of effectively modulating brain activity and, therefore, influencing cognitive processes.
As mentioned above, the two approaches used today for the simulation of brain activity consist of spiking neural networks, where a set of equations are used to describe the activity of each individual neuron over time, and neural mass models. , where a reduced number of equations are used to describe the activity of an entire population over time. The two simulation techniques are used in a complementary way, to understand and reproduce the mechanisms underlying diseases of the central nervous system, through the study and reproduction of typical CF patterns, and their disruption, obtained from real brain data ( neurophysiological and structural).
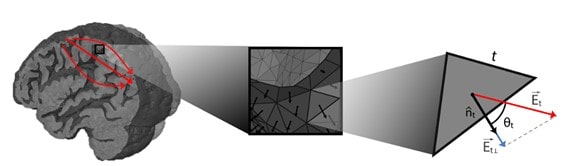
We can think of our brain as an electrochemical information processing system. When we apply tES using electrodes on the scalp, we induce weak electric fields in the head capable of altering the membrane potentials of neurons in the cerebral cortex. The effect is especially effective in the so-called pyramidal cells (a type of elongated neurons, aligned with each other, and perpendicular to the surface of the cortex) and depends on the relative orientation of the electric field vector (i.e., the E vector) with regarding the orientation of pyramidal cells. All of this is capable of modifying the way in which some parts of the brain process information. The change in member potential ana is the fundamental mechanism behind tES, and there are numerous research studies that quantify it (Ruffini et al. 2013).
In order to simulate a brain under stimulation, two types of computational models are normally used: current propagation models, which are designed to analyze, predict and regulate the electric fields generated in the brain during stimulation, taking into account factors such as the conductivity and shapes of brain tissues, and the configuration of the electrodes or coils used for stimulation. On the other hand, neuronal activation models focus on understanding and predicting how brain activity is modified, taking into account the biophysical characteristics of neurons. Together, these computational models provide valuable information about the effects and mechanisms of brain stimulation.
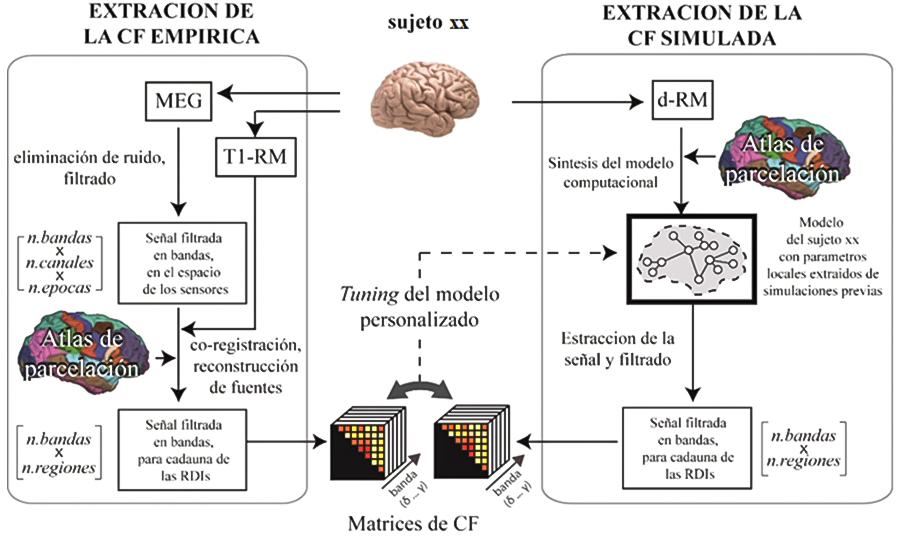
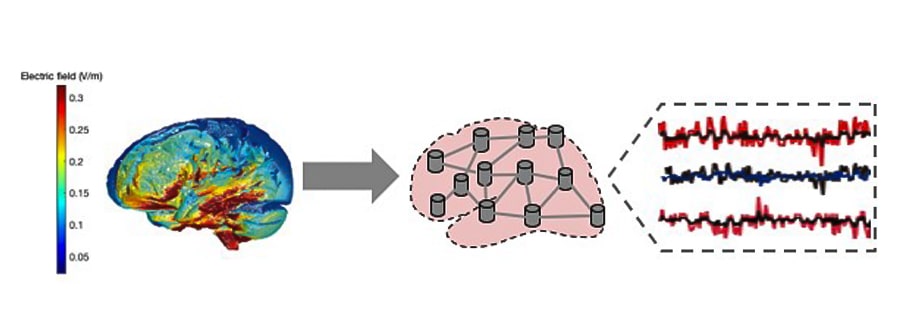
For the current propagation models we used magnetic resonance images (MRI-T1) of the participants to segment the brain tissues and generate a personalized volumetric mesh, which allows us to estimate the propagation of the electric field through the brain under stimulation at each point. of the brain (Huang et al. 2018; Merlet et al. 2013)On the other hand, information on the orientation of the neuronal axis of the pyramidal cells is obtained using a superficial mesh of the boundary surface between the white matter and the gray matter.
All this allows us to calculate the values of the projections of the electric field in the normal direction with respect to each portion of the surface. In cases where the fields are aligned with the orthodromic direction (from the apex towards the axon) of the neurons, a positive variation in the membrane potential will occur, unlike those in the antidromic direction, which will produce a negative variation in the potential. membrane (Merlet et al. 2013)For each subject, the projections of the electric fields can be grouped, obtaining the effect on each of the regions. As a final point, these data are introduced into the neuronal activation models (based on the SNN or NMM approaches already discussed above) and a calibration procedure is carried out to find the scale factor that maximizes the coincidence with the empirical observations. At this point we have all the ingredients for an in-silico reproduction of the brain under stimulation (Figure 4).
The clinically relevant effects of tES are related to changes in cortical excitability and brain connectivity. It is interesting to note that, in addition to inducing temporary effects, non-invasive brain stimulation techniques are capable of interacting with natural plasticity mechanisms, causing long-lasting effects (Huang et al. 2017; Frase et al. 2021; Nitsche and Paulus 2000)The mechanism underlying the permanence of these modifications can be found in an important phenomenon described by Donald Hebb: considering a pair of neurons connected through a synapse, if the presynaptic neuron persistently participates in making the postsynaptic neuron fire, the connection between them will gradually strengthen. And vice versa, if the activity of these two neurons is not causally related, they will gradually disconnect. It is the modification between neuronal connections that makes the effects last after stimulation. The effects caused by these techniques depend on the parameters of the stimulation, which include intensity, duration and frequency.
Modeling Attention Deficit Hyperactivity Disorder (ADHD)
Our multiscale modeling provides a unique opportunity to interpret the mechanisms of this disease, and understand which variables can be modulated in real subjects, and how to do so, to restore brain network functioning that is as similar to that of healthy people. We are developing the first 2 phases of this line, which we describe below:
PHASE 1 – In-silico reproduction of two groups of real subjects: ADHD group and control group.
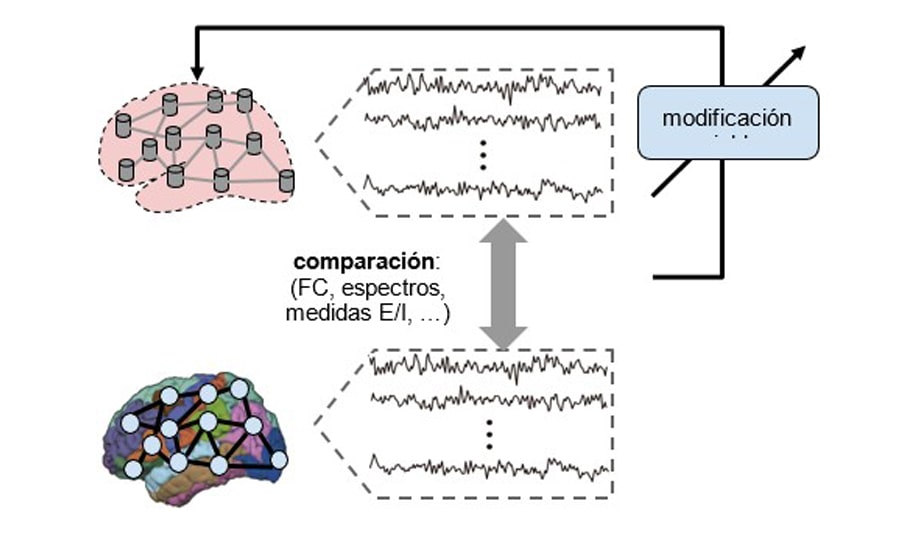
Search for the working point of the computational model.
This first step, which serves to adjust the computational model to the empirical observations, consists of determining the optimal value of a set of internal parameters of the model, which determines a simulated activity that is as similar as possible to the experimental data. The evaluation of the similarity between the two, is carried out by calculating specific metrics (e.g., functional connectivity, spectral profile, criticality measures), both in the real case and in the simulated case and evaluating the error or similarity between them. In previous work on ADHD, among the internal variables to vary for this purpose, the global coupling parameter, the bifurcation parameter, the modulation index, and the feedback coefficient have been considered (Iravani et al. 2021).
To this end, we propose a multi-dimensional tuning strategy including metrics of excitation/inhibition balance and brain criticality (Bruining et al. 2020; Simola et al. 2017) to contemplate the peculiarities recently found in ADHD subjects (Karalunas et al. 2022).
Neurophysiological techniques: a direct and non-invasive measurement of the time course of brain activity.
Another fundamental characteristic to faithfully reproduce the brain mechanisms of the real subject is the type of data available, and especially the neuroimaging technique in use for the experimental study. The neurophysiological techniques EEG and MEG (electroencephalography and magnetoencephalography, respectively), in addition for being non-invasive, are based on the direct measurement of potentials and fields produced by the activation of neurons present in the areas of the brain underlying the sensors used to the detection. In contrast to neurophysiological techniques, there are other techniques that are used for the same objectives, such as fMRI, which provide indirect measures of the underlying neuronal activity (contrast oxygen level-dependent imaging – or BOLD), and whose results should be interpreted carefully.
The good compromise between temporal and spatial resolution that characterizes MEG allows us to faithfully reconstruct the signal generated by neuronal populations from external measurements. The combined use of EEG, which measures the same type of signal with cheaper technology, allows the findings to be transferred to the clinical field, or to implement detection methods in portable setups.
In this study, both MEG and EEG will be used, and participants will also undergo structural magnetic resonance imaging (s-MRI) to correctly process the data obtained with the aforementioned functional techniques (resolution of the inverse problem and reconstruction of the signal at the level of the cortical regions of interest – or ROIs).
100% personalized models.
The structural connectivity, on which the local activity generators will be mounted, will be obtained by diffusion weighted magnetic resonance imaging (dw-MRI) from the same study subjects, and will not be imported from databases. This will allow us to generate highly personalized models that will allow to accurately answer the questions that may arise in the analysis of specific subjects. In fact, although some studies do not report significant changes in the structure of white matter between healthy subjects and ADHD, in general, computational modeling studies indicate that it is important to take into account the structural variability of the individual (essentially the number of brain tracts) white matter and its lengths when generating the associated computational models.
Multiscale approach for simulation and inspection.
Unlike NMMs, SNNs give us the possibility of evaluating the dynamics of the simulated network at different levels of activity. Thanks to the Institute’s computing resources (including GPUs for parallel processing) it is possible to simulate the activity produced by the ROIs with models based on spiking neurons, in affordable times. Realistic neural interconnections, based on the real structure of the cortical columns (Potjans and Diesmann 2014)and the interconnection between them based on the structural connectivity of the subject to be simulated, obtained by dw-MRI (Susi et al. 2021)ensure high biological plausibility. In addition, selective changes can be introduced at different scales, even at the neuron level, modeling even molecular mechanisms including the action of drugs. Likewise, the repercussions can be observed not only at the macro and mesoscale, but also at the neuron level (Figures 5 and 6).

PHASE 2 – Design and optimization of transcranial brain stimulation to induce optimal balance and analyze the evolution of the network after stimulation.
Although transcranial stimulation techniques appear to show promise in improving ADHD deficits, it is still not possible to precisely determine the clinical usefulness. Cortical regions involved in the pathophysiology of ADHD, stimulation parameters (e.g., intensity, duration, polarity, and electrode size), and types of symptoms/deficits are potential determinants of the efficacy of transcranial stimulation in ADHD (Salehinejad et al. 2020).
Our computational models will allow us to find the optimal configuration and duration of the stimulation to be performed, and at the same time predict if the results have been achieved. It should be noted that unlike NMMs, SNNs allow us to better model the stimulation heterogeneity in the cortical blanket, a consequence of the orientation of the gray matter with respect to the electric field lines induced in the brain (Cabrera-Álvarez et al. 2023)and evaluate the action of synaptic plasticity on the evolution of network parameters after stimulation (Figure 7).
Bruining, Hilgo, Richard Hardstone, Erika L. Juarez-Martinez, Jan Sprengers, Arthur-Ervin Avramiea, Sonja Simpraga, Simon J. Houtman, et al. 2020. “Measurement of Excitation-Inhibition Ratio in Autism Spectrum Disorder Using Critical Brain Dynamics.” Scientific Reports 10 (1): 9195.
Cabrera-Álvarez, Jesús, Jaime Sánchez-Claros, Martín Carrasco-Gómez, Alberto Del Cerro-León, Carlos J. Gómez-Ariza, Fernando Maestú, Claudio R. Mirasso, and Gianluca Susi. 2023. “Understanding the Effects of Cortical Gyrification in tACS: Insights from Experiments and Computational Models.” Frontiers in Neuroscience 17 (August): 1223950.
Frase, Lukas, Lydia Mertens, Arno Krahl, Kriti Bhatia, Bernd Feige, Sven P. Heinrich, Stefan Vestring, et al. 2021. “Transcranial Direct Current Stimulation Induces Long-Term Potentiation-like Plasticity in the Human Visual Cortex.” Translational Psychiatry 11 (1): 17.
Huang, Ying-Zu, Ming-Kue Lu, Andrea Antal, Joseph Classen, Michael Nitsche, Ulf Ziemann, Michael Ridding, et al. 2017. “Plasticity Induced by Non-Invasive Transcranial Brain Stimulation: A Position Paper.” Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology 128 (11): 2318–29.
Huang, Yu, Abhishek Datta, Marom Bikson, and Lucas C. Parra. 2018. “ROAST: An Open-Source, Fully-Automated, Realistic Volumetric-Approach-Based Simulator For TES.” Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2018 (July): 3072–75.
Iravani, Behzad, Artin Arshamian, Peter Fransson, and Neda Kaboodvand. 2021. “Whole-Brain Modelling of Resting State fMRI Differentiates ADHD Subtypes and Facilitates Stratified Neuro-Stimulation Therapy.” NeuroImage 231 (May): 117844.
Karalunas, Sarah L., Brendan D. Ostlund, Brittany R. Alperin, Mckenzie Figuracion, Hanna C. Gustafsson, Erika Michiko Deming, Dan Foti, et al. 2022. “Electroencephalogram Aperiodic Power Spectral Slope Can Be Reliably Measured and Predicts ADHD Risk in Early Development.” Developmental Psychobiology 64 (3): e22228.
Merlet, Isabelle, Gwénaël Birot, Ricardo Salvador, Behnam Molaee-Ardekani, Abeye Mekonnen, Aureli Soria-Frish, Giulio Ruffini, Pedro C. Miranda, and Fabrice Wendling. 2013. “From Oscillatory Transcranial Current Stimulation to Scalp EEG Changes: A Biophysical and Physiological Modeling Study.” PloS One 8 (2): e57330.
Nitsche, M. A., and W. Paulus. 2000. “Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation.” The Journal of Physiology 527 Pt 3 (Pt 3): 633–39.
Potjans, Tobias C., and Markus Diesmann. 2014. “The Cell-Type Specific Cortical Microcircuit: Relating Structure and Activity in a Full-Scale Spiking Network Model.” Cerebral Cortex 24 (3): 785–806.
Ruffini, Giulio, Fabrice Wendling, Isabelle Merlet, Behnam Molaee-Ardekani, Abeye Mekonnen, Ricardo Salvador, Aureli Soria-Frisch, Carles Grau, Stephen Dunne, and Pedro C. Miranda. 2013. “Transcranial Current Brain Stimulation (tCS): Models and Technologies.” IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society 21 (3): 333–45.
Salehinejad, Mohammad Ali, Vahid Nejati, Mohsen Mosayebi-Samani, Ali Mohammadi, Miles Wischnewski, Min-Fang Kuo, Alessio Avenanti, Carmelo M. Vicario, and
Michael A. Nitsche. 2020. “Transcranial Direct Current Stimulation in ADHD: A Systematic Review of Efficacy, Safety, and Protocol-Induced Electrical Field Modeling Results.” Neuroscience Bulletin 36 (10): 1191–1212.
Simola, Jaana, Alexander Zhigalov, Isabel Morales-Muñoz, J. Matias Palva, and Satu Palva. 2017. “Critical Dynamics of Endogenous Fluctuations Predict Cognitive Flexibility in the Go/NoGo Task.” Scientific Reports 7 (1): 2909.
Susi, Gianluca, Pilar Garcés, Emanuele Paracone, Alessandro Cristini, Mario Salerno, Fernando Maestú, and Ernesto Pereda. 2021. “FNS Allows Efficient Event-Driven Spiking Neural Network Simulations Based on a Neuron Model Supporting Spike Latency.” Scientific Reports 11 (1): 12160.
Cabrera-Álvarez, Jesus, Jaime Sánchez-Claros, Martín Carrasco-Gómez, Alberto del Cerro-León, Carlos J. Gomez-Ariza, Fernando Maestú, Claudio Mirasso, Gianluca Susi “Understanding the Effects of Cortical Gyrification in tACS: Insights from Experiments and Computational Models”. Frontiers in Neuroscience (In publication)